Reversible Crosslinking: a potent paradigm for designer materials (TU/e)
- Project leaders: prof.dr. Kees Storm (TU/e) & dr. Wouter Ellenbroek (TU/e), Department of Applied Physics, Theory of Polymers and Soft Matter
- Postdoc: dr. Nicholas B. Tito (now R&D Scientist at Electric Ant Lab, Amsterdam)
- International expert: dr. Costantino Creton (ESPCI ParisTech)
Awards
 Nicholas B. Tito has been showcased a '2019 Emerging Leader', for the Journal of Physics: Condensed Matter. This involved contributing an invited peer-reviewed article for the journal: 'First-order 'hyper-selective' binding transition of multivalent particles under force' [Tine Curk and Nicholas B. Tito 2020 J. Phys.: Condens. Matter 32 214002].
Nicholas B. Tito has been showcased a '2019 Emerging Leader', for the Journal of Physics: Condensed Matter. This involved contributing an invited peer-reviewed article for the journal: 'First-order 'hyper-selective' binding transition of multivalent particles under force' [Tine Curk and Nicholas B. Tito 2020 J. Phys.: Condens. Matter 32 214002].
Emerging Leaders 2019 - Journal of Physics: Condensed Matter
(Photo by: Ernst de Groot)
Interview
'Entropy: friend or foe of the materials scientist' (4TU.HTM publication, Pdf file)
 There is an intriguing polymer class that contains reversible crosslinks, mobile connections between two polymer chains that can easily be reattached when broken. Nicholas B. Tito was fascinated by the effects of these crosslinking additives on the overall material properties, because of the prospects of making it into recyclable or self-repairing materials. Whereas adding more crosslinks into a polymer network usually causes it to become stiffer, less elastic and more brittle, experiments show that the addition of certain kinds of reversible crosslinks toughens the material without compromising its stretchability. Trying to understand this was the starting point of the project ‘Reversible Crosslinking’. Nicholas worked with several simulation and numerical modelling techniques. What this 4TU.HTM project has yielded is a microscopic design concept and modelling strategies for building this kind of self-repairing materials in which entropy ensures that the polymers constantly want to explore new connections. Because of this the researchers expect to discover new materials with special properties.
There is an intriguing polymer class that contains reversible crosslinks, mobile connections between two polymer chains that can easily be reattached when broken. Nicholas B. Tito was fascinated by the effects of these crosslinking additives on the overall material properties, because of the prospects of making it into recyclable or self-repairing materials. Whereas adding more crosslinks into a polymer network usually causes it to become stiffer, less elastic and more brittle, experiments show that the addition of certain kinds of reversible crosslinks toughens the material without compromising its stretchability. Trying to understand this was the starting point of the project ‘Reversible Crosslinking’. Nicholas worked with several simulation and numerical modelling techniques. What this 4TU.HTM project has yielded is a microscopic design concept and modelling strategies for building this kind of self-repairing materials in which entropy ensures that the polymers constantly want to explore new connections. Because of this the researchers expect to discover new materials with special properties.
Stretching the boundaries of materials. Six projects of the 4TU.HTM programme 'New Horizons in Designer Materials', p.54-62, March 2020.
Papers
'First-order 'hyper-selective' binding transition of multivalent particles under force' by Nicholas B. Tito and Tine Curk.
[Journal of Physics: Condensed Matter, 2020, 32, 214002]
'Multivalent “attacker and guard” strategy for targeting surfaces with low receptor density' by Nicholas B. Tito (TU/e).
[The Journal of Chemical Physics 150, 184907 (2019)]
‘Harnessing entropy to enhance toughness in reversibly crosslinked polymer networks’ by Tito, N. B., Creton, C., Storm, C., Ellenbroek, W. G.
[Soft Matter, 15, 2190-2203, 2019]
 'Embracing Entropy in the Design of New Materials' by Nicholas B. Tito (TU/e).
'Embracing Entropy in the Design of New Materials' by Nicholas B. Tito (TU/e).
[Innovative Materials, 2018, 2, pp. 28-31.]
In Dutch: 'Omarm entropie in het ontwerpen van nieuwe materialen' door Nicholas B. Tito (TU/e). [Innovatieve Materialen, 2018, 2, pp. 32-35.]
'Self-Consistent Field Lattice Model for Polymer Networks' by dr. Nicholas B. Tito, dr. W. Ellenbroek and prof.dr. Kees Storm (TU/e).
[Macromolecules, 2017, 50 (24), pp 9788-9795]
'Protruding organic surfaces triggered by in-plane electric fields' by dr. Danqing Lui, dr. Nicholas B. Tito and prof. Dirk J. Broer (TU/e).
[Nat. Comm., 2017, 8, 1526.]
Presentation
'Exploiting entropy to enhance toughness in polymer gels with reversible crosslinks'
Presented by: Nicholas B. Tito (TU/e)
Date: Tuesday 14th May 2019 - 10:20 to 10:40
Event: Optimal design of soft matter - including a celebration of Women in Materials Science (WMS) at the Isaac Newton Institute.
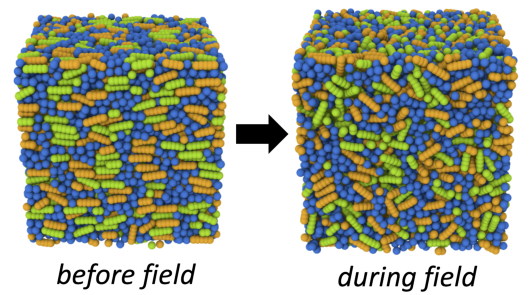
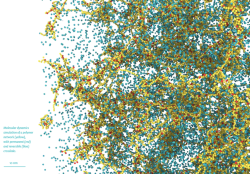
Image 1. Liquid Crystals (incl. movie)
Molecular dynamics simulations of electrically-responsive liquid crystalline species cross-linked into a glassy polymer network. The polymer monomers are shown in blue, and the liquid crystals are colored in orange and green. Green molecules are dipolar, and therefore respond to an applied electric field; orange molecules are passive. By applying an AC electric field to the molecularly aligned sample, the active molecules are torqued, local molecular order is disrupted, and the local system volume increases (left to right frame).
On a macroscopic scale, tuning which portion of the system is exposed to an electric field allows one to create user-defined surface topographies, useful for haptic feedback or self-cleaning surfaces on solar panels. Molecular simulation allows us to gain insight into the microscopic mechanism at play in the material, and how to tune the response of the material to an electric field based on chemical architecture.
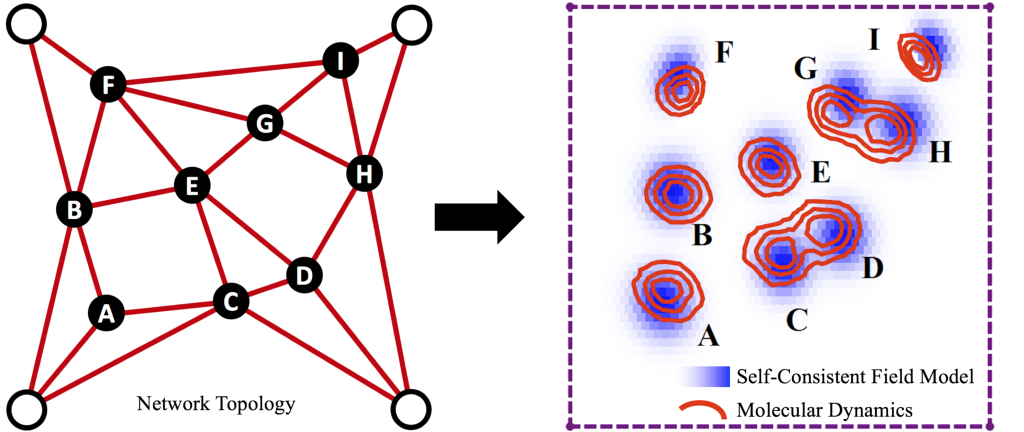
Image 2a. Network Structure
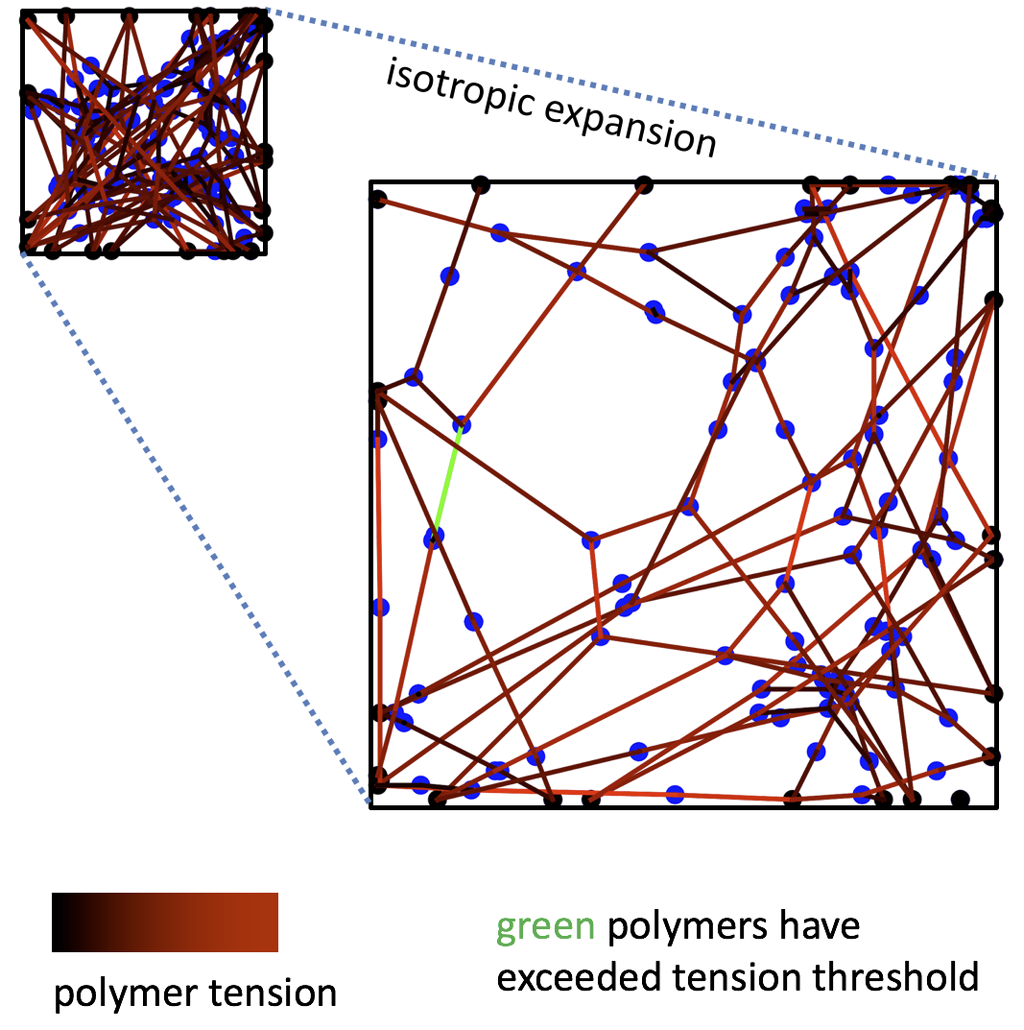
Image 2b. Network Deformation
At the microscale, polymer networks contain many macromolecules connected together by crosslinks into some topology. The spatial distributions of polymer chains and crosslinks in the sample is highly non-trivial depending on this topology, especially when the material is strained and polymers or crosslink bonds begin to break.
We are developing a molecular-scale model that gives us insight into polymer network structure, but without the computational complexity of a full molecular simulation. By taking an input network topology (a, left), the model uses “self-consistent field theory” to approximate the resulting spatial distributions of polymers and crosslinks in the sample. Among other things, the model outputs the “clouds” that crosslinks in the network trace out as they fluctuate at equilibrium (a, right). The size and shapes of these clouds reveal which parts of the network are floppy (high in entropy) or rigid (low in entropy). We can then deform the polymer network (b), and the model estimates which polymer chains will detach from their crosslinks as the material becomes highly strained.
In 2a: (left) Lines represent polymer chains connecting crosslinks (solid points). Open points are spatially-fixed “fence posts” to which the network is connected, so that it is under overall tension. (right) Blue shading are “clouds” for each crosslink obtained from our model, and red contours are the clouds obtained from molecular dynamics simulations for the same system.
In 2b: Black points represent crosslinks fixed to the square frame of the network (solid black outline), and blue points are “free” crosslinks. Lines represent polymer chains connecting the crosslinks, and red shading indicates how much tension the polymer is bearing. Green polymers have exceeded the tension threshold for breaking.
Abstract
The materials that our bodies are made of are remarkable. Our skin, cartilage, blood vessels, heart valves: all are 100% biobased, autonomously assembled, renewable, strong and self-healing. We literally trust these materials with our lives.
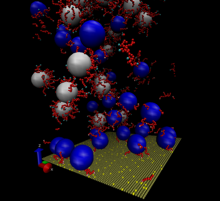
Image 3.
To develop the next generation of consumer polymeric materials requires innovative, nontraditional design approaches suitable for novel high-‐tech materials. While most agree that the key to addressing this challenge is to borrow design concepts from natural materials to enhance the performance of synthetics, few specific examples exist. In this proposal, we put forth a line of research that seeks to address the fundamental physics of one particularly potent, bio-insipred design feature, that is shared by intra-‐ and extracellular biomaterials: reversible crosslinking.
Reversibly crosslinked materials (RCM’s) are polymer networks, held together by links that can bind and unbind reversibly. The resulting dynamic material derives its strength, toughness and adaptability from a combination of physical and chemical dynamic linkage, which puts these materials, structurally, somewhere between classical thermoplasts and thermosets. But, in stark contrast to either of these materials, RCM’s “set” reversibly: under the influence of temperature, stress or strain binding and unbinding kinetics may be altered, resulting in highly tunable mechanical properties, enhanced toughness and recyclability. RCM’s combine the versatility in processing of thermoplastic materials with the high performance mechanics of thermosets. The fact that microscopically what was once loose may become bound, and vice versa, introduces a host of possibilities for microscopic stress release, strain distribution, shape adjustment and recovery after failure. In the first project of what we hope to grow into a thriving research line in Eindhoven, we drill down into what makes it work, and how we may make maximal use of it, for a specific experimental system, proven to respond strongly to reversible linkage. The research is jointly hosted by the Institute for Complex Molecular Systems and the Department of Applied Physics, which commits itself to support this line of research if it proves successful.
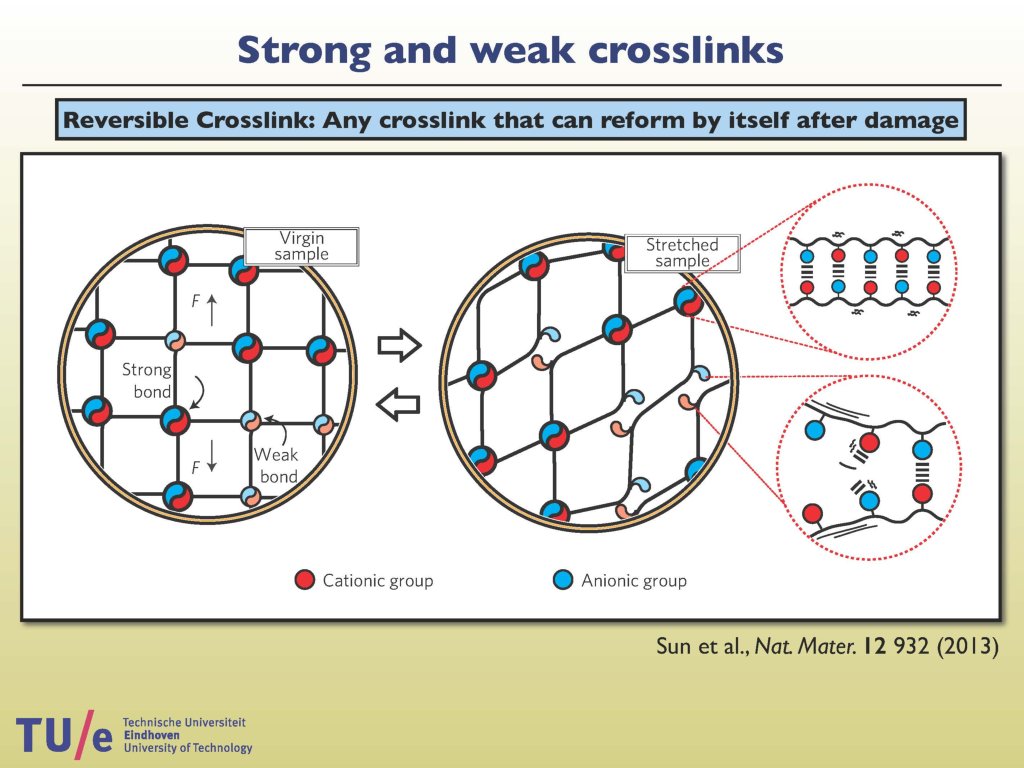
Presentation (Pdf file, 7 MB) held by dr. Wouter Ellenbroek at the Kick-off Symposium 'Dutch Materials 2015', 6 October 2015.
Presentation (Pdf file, 3 MB) held by dr. Nick Tito for 4TU.HTM, 29 September 2016.
Bio Postdoc Nick Tito
Growing up on the coast of Maine in the United States, Nick was inspired to study physical sciences by the powerful winter cyclones that strike his home town each year. Northeastern snowstorms are a rare example of how physics through a continuum of scales---microscopic to global---act in coincidence to form a beautiful, yet fleeting, natural structure.
Building on this inspiration, Nick enjoys solving challenging problems in chemistry and physics, with the goal of discovering new mathematical and physical beauty in nature.
Nick did his PhD at Dartmouth College (New Hampshire, USA) with Profs. Jane Lipson and Scott Milner, studying copolymer phase behaviour and the glass transition in thin polymer and liquid films. He then carried out post-doctoral research as a Marie Curie Innovative Training Network experienced researcher at Cambridge University (Cambridge, UK), with Prof. Daan Frenkel. There, his work examined how “multivalent” design strategies can be employed to tune the binding sensitivity of polymers and nanoparticles to other microscopic entities.
Currently, Nick is a post-doctoral researcher at University of Technology, Eindhoven (Eindhoven, Netherlands), with Profs. Wouter Ellenbroek and Cornelis Storm. Funded by the 4TU.HTM “New Horizons in Designer Materials” program, Nick is studying how to optimize the properties of polymeric materials with dynamic reversible microscopic interactions.

Photo: Nicholas B. Tito (by Ernst de Groot)



